| SCDigest Says: |
 Rogers believes a variety of dynamics will mean manufacturers will comply with the California law by using 2D codes rather than RFID. Rogers believes a variety of dynamics will mean manufacturers will comply with the California law by using 2D codes rather than RFID.

Click Here to See Reader Feedback |
A variety of regulatory forces will push the pharmaceutical supply chain to adopt serialization of drugs at the unit level and so-called “electronic pedigrees” used to track a pharmaceutical product’s full custodial lifecycle.
These requirements, some of which have already become mandates at the state level such found in California’s e-pedigree legislation, mean that starting at the manufacturer, unit-level pharmaceutical products will need to carry some form of automatic identification.
Not long ago, most in the industry assumed that would come in the form of RFID tags. Now, one pharma industry pundit says the victor is more likely to be two-dimensional bar codes – offering insight into the cost dynamics that may be noteworthy to those in any industry.
Dirk Rogers is a long-time pharmaceutical industry IT consultant, as well as co-chair of the GS1 EPCglobal Drug Pedigree Messaging work group and is a member of many other GS1 and GS1 U.S work groups.
In a recent article, Rogers says that the reality of costs, especially initial investments across the supply chain, likely means that 2D codes will win out to achieve serialization and pedigree tracking in pharma.
The graph below shows Rogers’ relative estimate of the net costs (translate to “ reverse ROI”) for manufacturers, wholesalers, and retailers for investment in RFID to meet the new requirements. These graphs include the important assumption that all prescription pharmaceutical sellable packages are tagged with GS1 Gen2 RFID tags with a Serialized Global Trade Item Number (SGTIN) encoding.
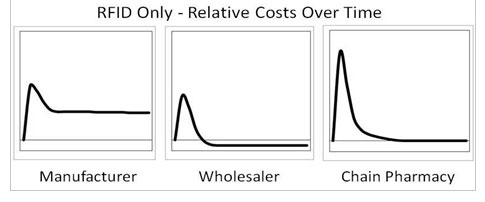
Manufacturers have high initial and on-going costs because they will have to have these systems pass FDA validation and will bear the cost of on-going tagging. Wholesalers benefit the most, showing positive ROI not long down the road, because they can leverage the tagged products for internal DC operations with relatively little on-going investment and almost no on-going costs. Retailers would have major initial costs due to the need to outfit hundreds or thousands of outlets with RFID reading systems.
2D a Less Expensive Alternative?
Rogers then created parallel graphs if 2D codes were used instead of RFID, as shown below.
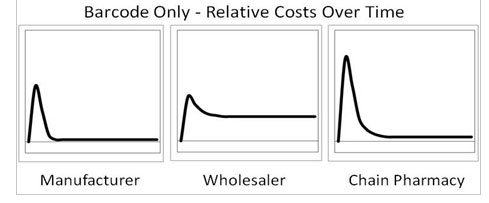
“The post-deployment costs for a pharmaceutical manufacturer and wholesaler have flipped compared with RFID,” Rogers says. “With barcodes, a manufacturer’s recurring costs drop considerably because ink for barcodes is a lot cheaper than RFID tags. But now the wholesaler’s long-term costs are higher because they can’t read every serial number without manually manipulating each unit and aiming a barcode reader directly at the barcode that contains the serial number. This will make the receiving and order fulfillment operations much less efficient.”
(RFID and Automatic Identification Article - Continued Below)
|